About pharmaceutical innovation
Pharmaceutical innovation is the process aimed at developing a new active substance that does not yet exist for the treatment of a specific disease and that provides a novel therapeutic benefit, as well as ensuring its effective and safe application.
As a result of this process, innovative pharmaceutical companies offer concrete solutions to previously unmet medical needs, contribute to the expansion of the pharmacopeia, and drive progress in medical treatment. Innovative medicines represent the most advanced and effective solutions currently available for combating various diseases, thereby improving patients’ quality of life.
New Directions in Pharmaceutical Innovation
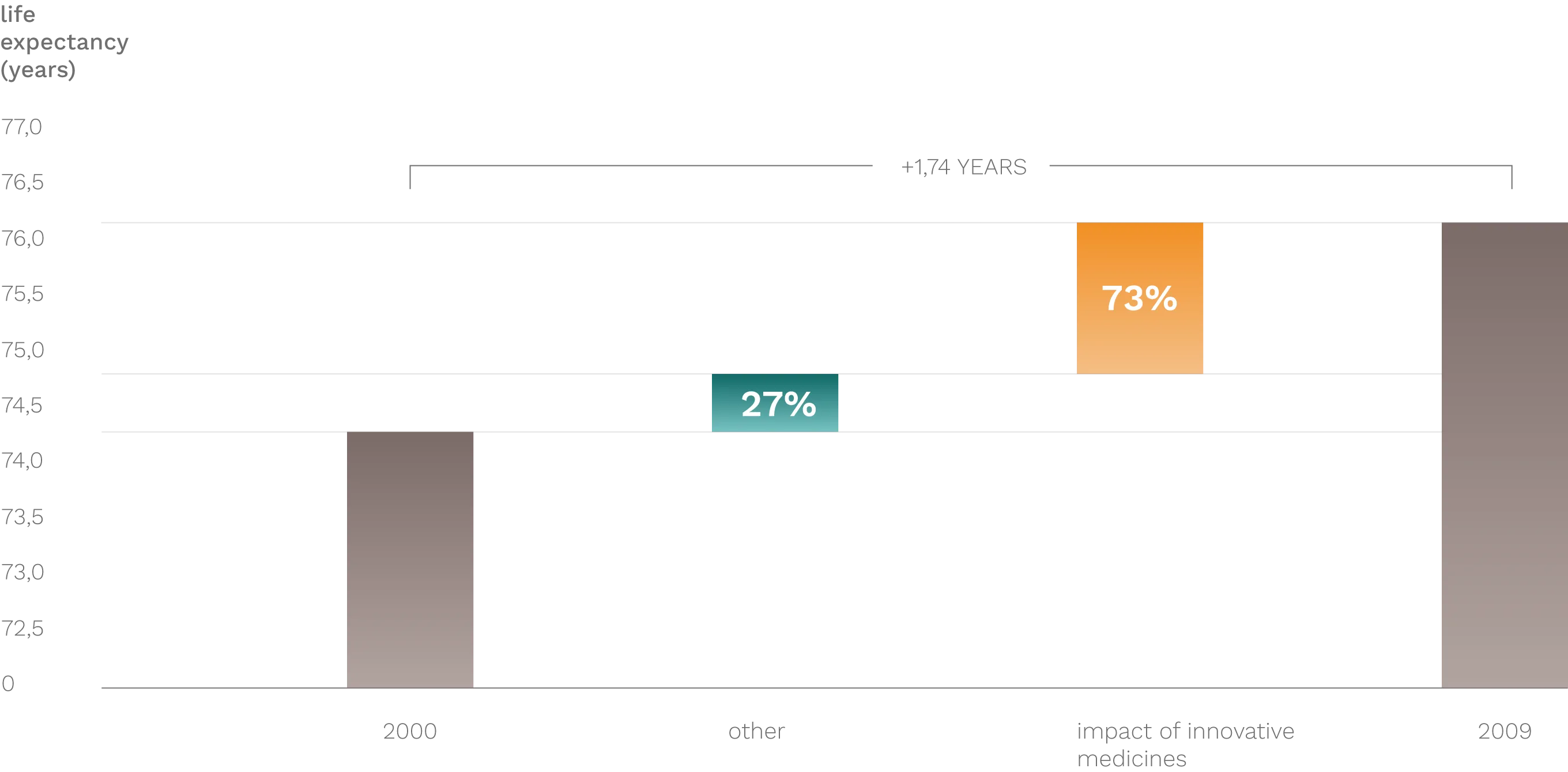
Over the past 30–40 years, numerous diseases (e.g., cardiovascular, oncological, psychiatric, metabolic, and pulmonary conditions, as well as AIDS) have become curable or more effectively manageable thanks to innovative medicines. As a result, both quality of life and life expectancy have increased to previously unimaginable levels. According to estimates, 73% of the rise in life expectancy can be attributed to innovative pharmaceuticals (source: Lichtenberg, F. (2012): Pharmaceutical Innovation and Longevity Growth in 30 Developing OECD and High-Income Countries, 2000–2009).
THE IMPACT OF INNOVATIvE MEDICINES ON LIFE EXPECTANCY GROWTH (2000-2009)
Source: Lichtenberg, F: Pharmaceutical innovation and longevity growth in 30 developing OECD and high-income countries, 2000-2009 (2012)
The Process of Pharmaceutical Innovation
Innovative pharmaceutical companies are continuously engaged in research and development activities. This demanding process — requiring immense amounts of work, time, and financial investment — ultimately aims to help us preserve our health for as long as possible, recover it as quickly as possible, or, in the case of irreversible diseases, to halt or slow the progression and deterioration of the condition. Pharmaceutical innovation is a long, high-risk, and costly endeavor — out of approximately 1,000,000 potential molecules, only one typically results in a marketable medicine. In 2012, the development cost of a single innovative drug was at least 300 billion HUF (approximately 1 billion USD at the time), and the process from discovery to market authorization takes around 10 years.
THE PROCESS OF DEVELOPING INNOVATIVE MEDICINES
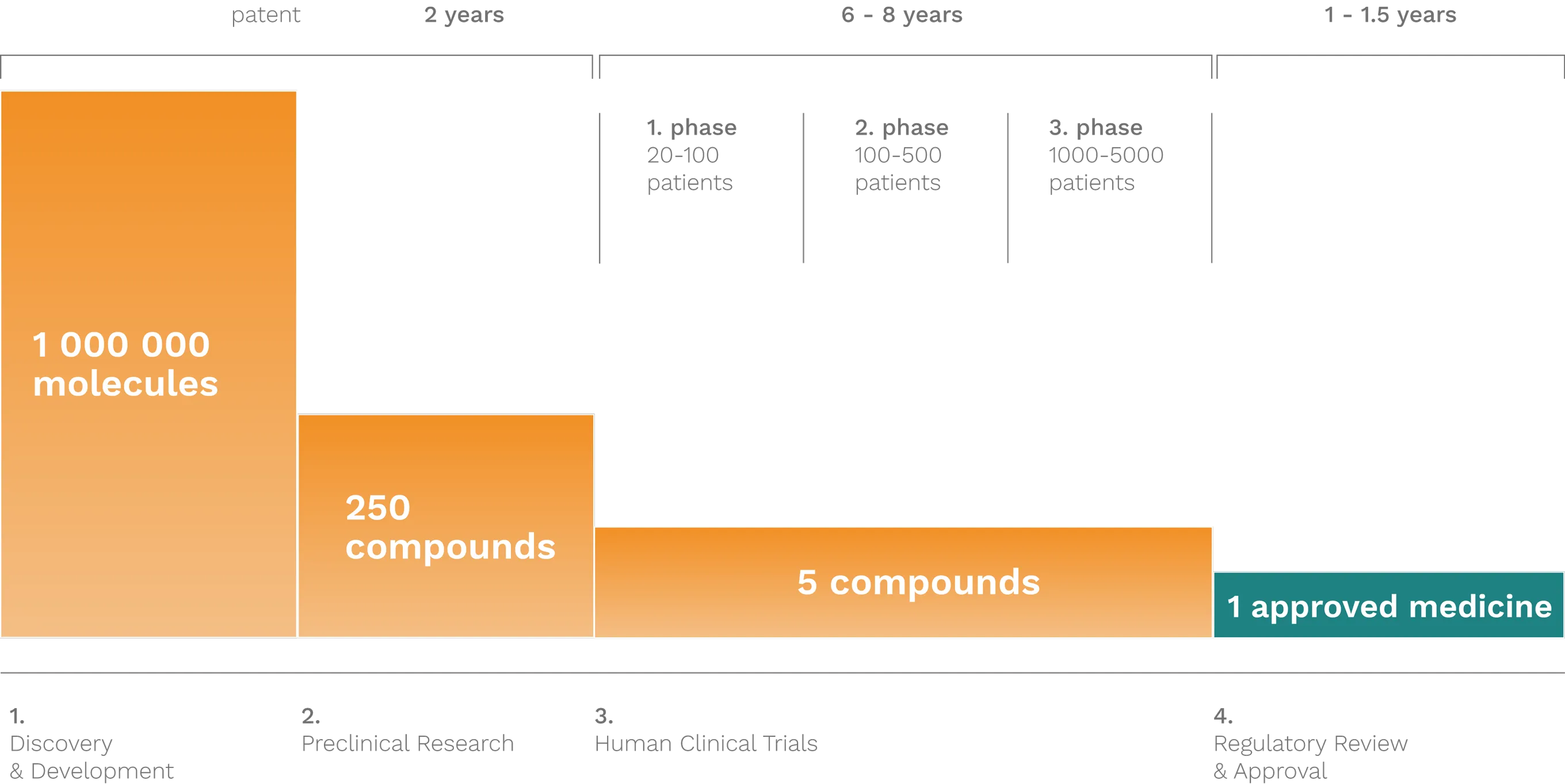
Innovative, or so-called originator active substances, are protected by patents at the time of their development. Patent protection typically lasts 20 years. While this may seem like a long period, drug development and regulatory approval processes take many years, meaning that by the time a medicine reaches the market, only around 10 years of patent protection often remain. Once the patent expires, copies of the medicine produced by other companies — known as generic drugs — can enter the market.
Although generics contain the same chemical active ingredient in the same quantity, there may be differences in the manufacturing process, the physical form of the drug, or its composition (e.g., excipients). Furthermore, the bioequivalence studies required to prove a generic drug’s similarity to the original product allow for some variation within an accepted range. These factors must be carefully considered when selecting the appropriate therapy for a patient.
The contribution of innovative pharmaceutical manufacturers in Hungary extends far beyond simply supplying medicines. Our member companies are major investors in both the economy and the healthcare sector, through conducting research and clinical trials in Hungary. In addition, they support continuing medical education, assist healthcare institutions by offering price discounts, and provide donations of medicines, equipment, and funds.
Economic Impact
Beyond these investments and contributions, innovative manufacturers create jobs, purchase goods and services, and generate income and added value within the national economy. Their presence and operations indirectly support the retention of further jobs and play a crucial role in helping retain highly qualified healthcare professionals in the country.
The direct and indirect economic impact of innovation is significant. Innovative pharmaceutical companies are among the top investors in research and development, reinvesting on average 15.1% of their revenues into R&D.
